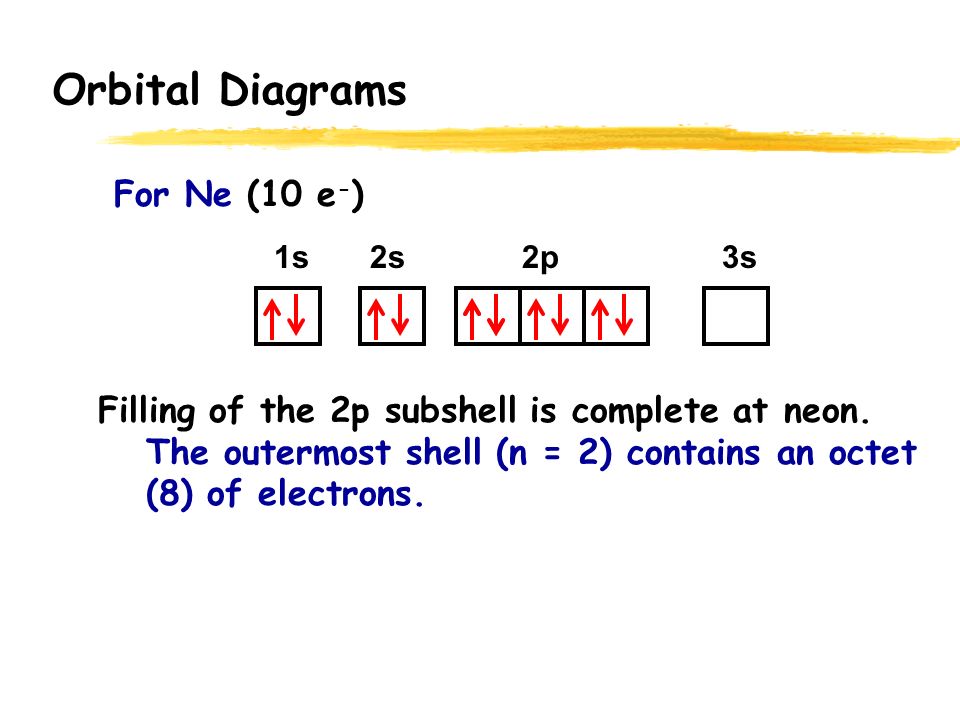
5+ orbital diagram for neon
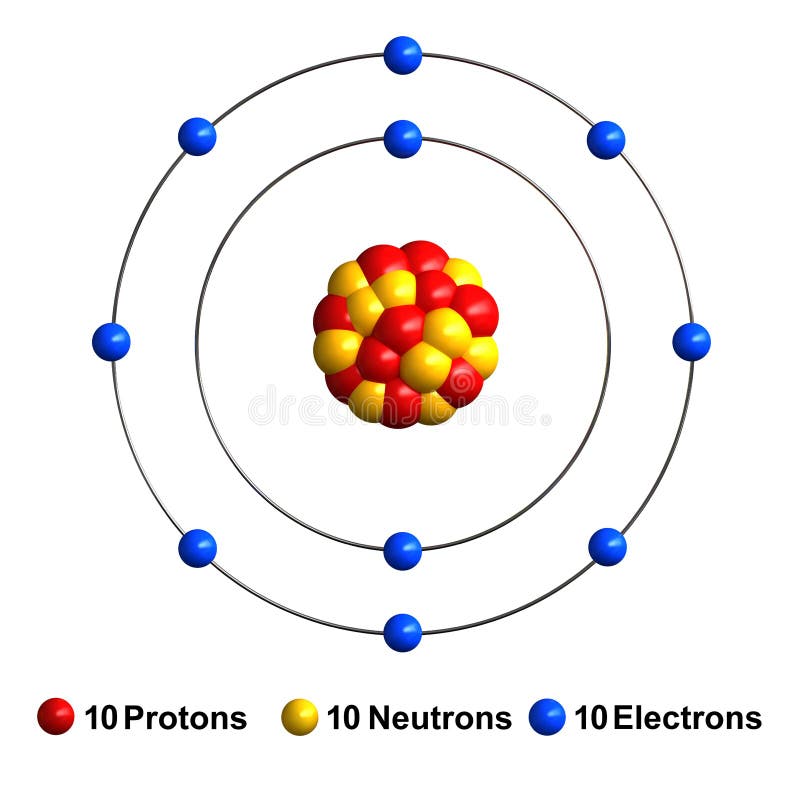
The concentration of water vapor a greenhouse gas varies significantly from around 10 ppm by mole fraction in the coldest portions of the atmosphere to as much as 5 by mole fraction in hot humid air masses and. An atom of the alkaline earth metal beryllium with an atomic number of 4 contains four protons in the nucleus and four electrons surrounding the nucleus.
Neon Atoms Stock Illustrations 378 Neon Atoms Stock Illustrations Vectors Clipart Dreamstime
This is called quantum jump.

. A nitrogen atom has seven electrons. Thus the electron configuration and orbital diagram of lithium are. In atomic physics the Bohr model or RutherfordBohr model presented by Niels Bohr and Ernest Rutherford in 1913 is a system consisting of a small dense nucleus surrounded by orbiting electronssimilar to the structure of the Solar System but with attraction provided by electrostatic forces in place of gravityIt came after the solar system Joseph Larmor model.
It is named after the Roman god Mercurius god of commerce messenger of the gods and mediator between gods and mortals corresponding to the Greek god Hermes Ἑρμῆς. ASCII characters only characters found on a standard US keyboard. For light atoms the spinorbit interaction or coupling is small so that the total orbital angular momentum L and total spin S are good quantum numbersThe interaction between L and S is known as LS coupling RussellSaunders coupling named after Henry Norris Russell and Frederick Albert Saunders who described this in 1925 or spin-orbit.
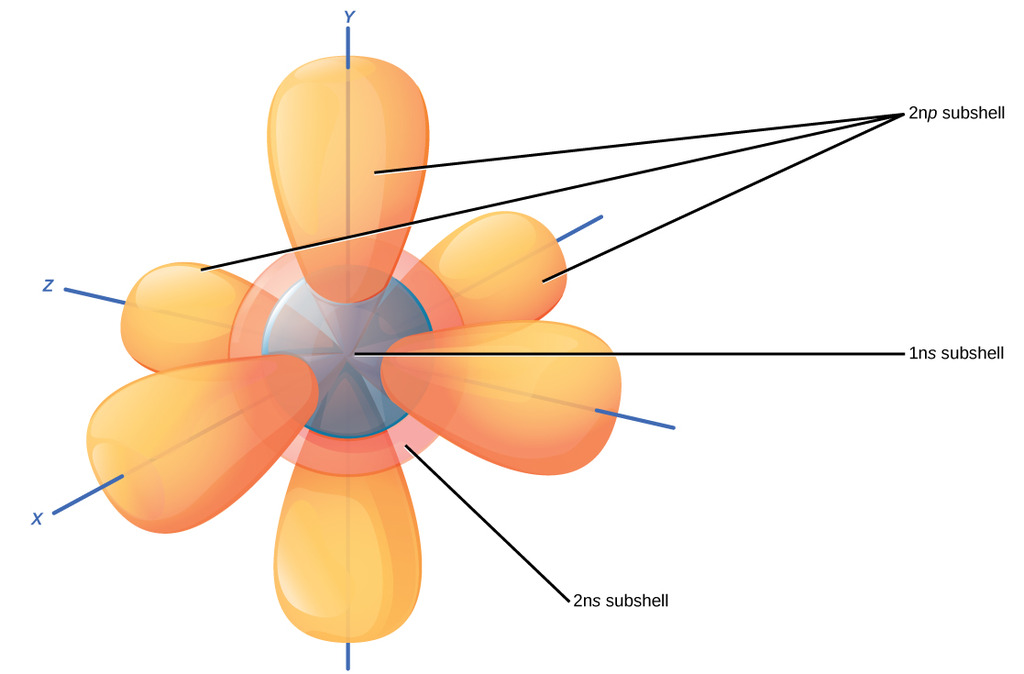
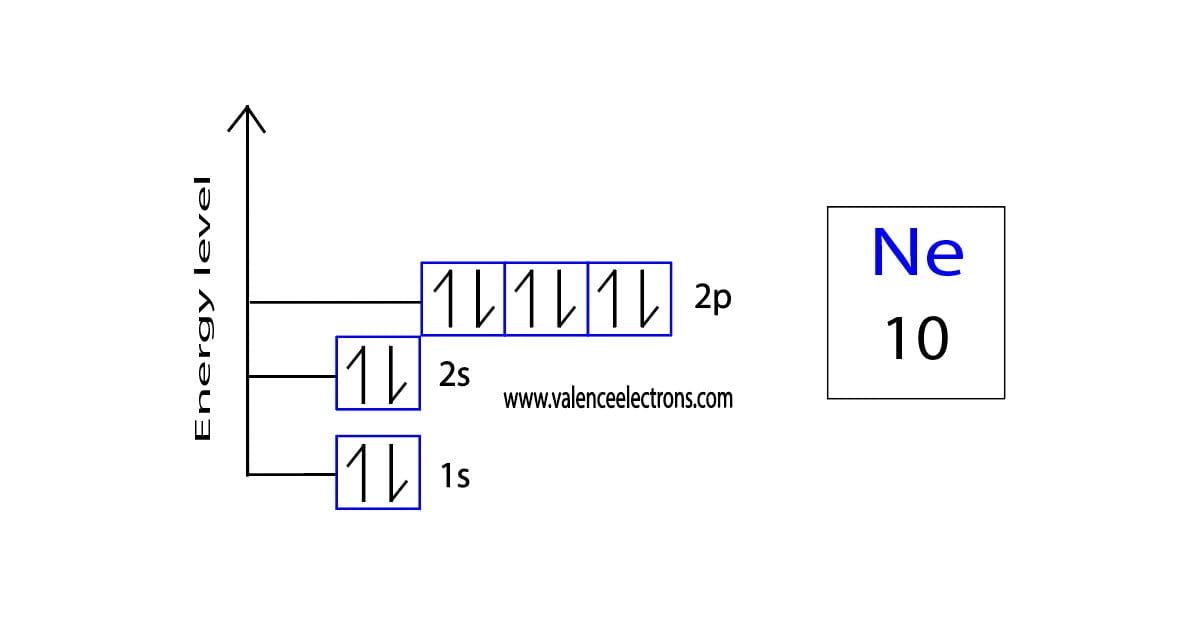
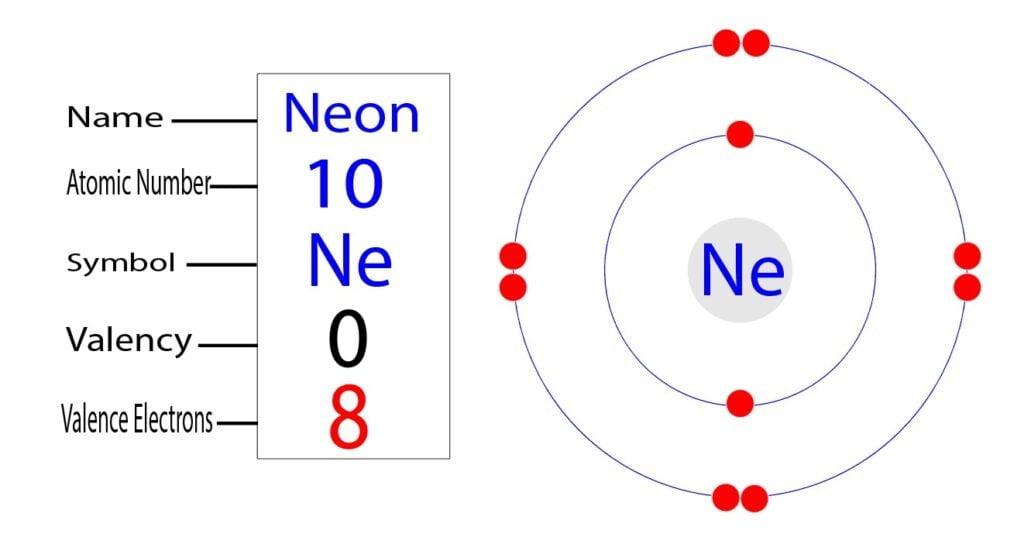
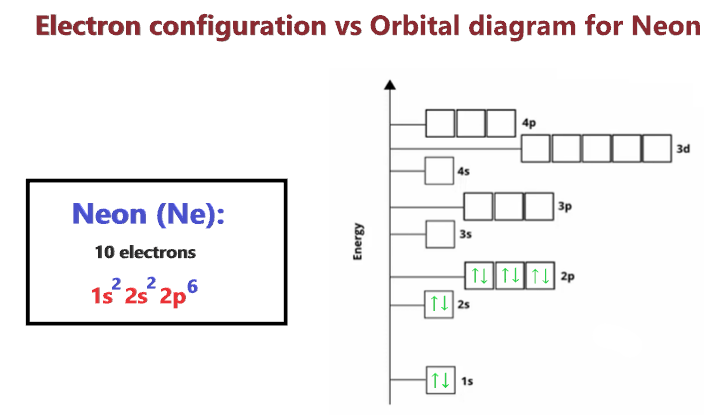
The short electron configuration of neon is 2s 2 2p 6. In the ground state they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 zIt therefore has five valence electrons in the 2s and 2p orbitals three of which the p-electrons are unpaired. The s-orbital can have a maximum of two electrons.
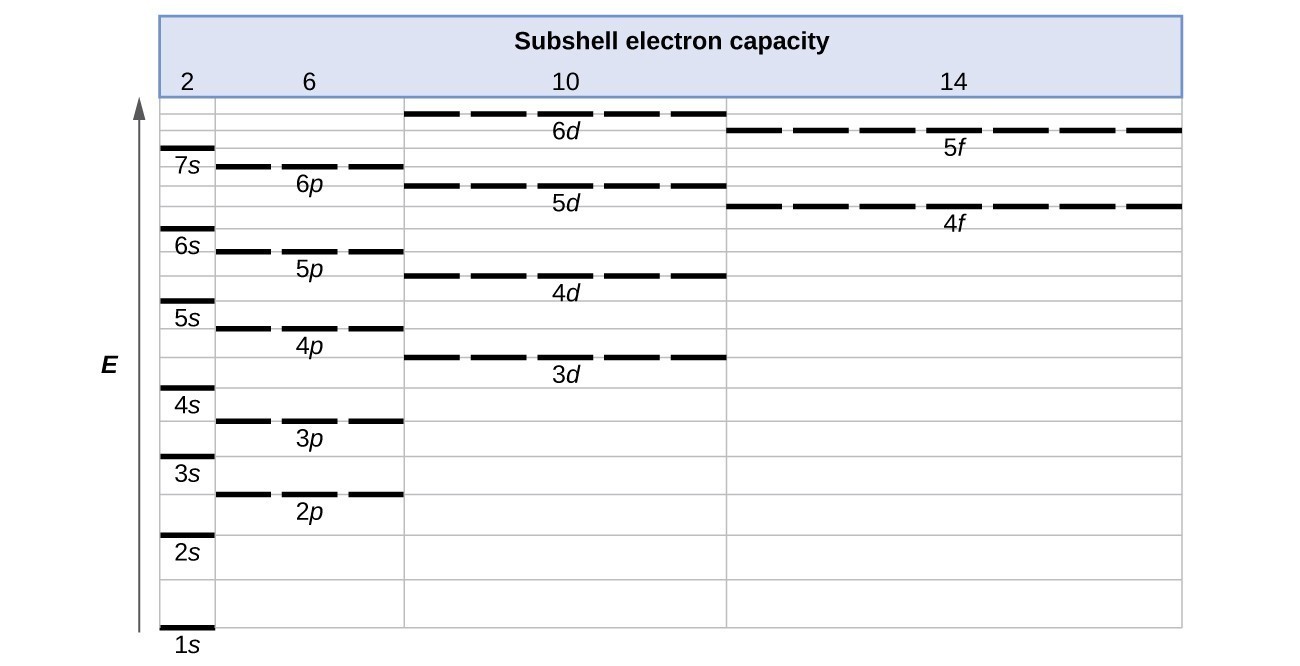
Like Venus Mercury orbits the Sun within. Here the energy of 4s orbital is less than that of 3d. So the remaining three electrons enter the 2p orbital.
The first ionization energy is quantitatively expressed as Xg energy X g e. The fourth electron fills the remaining space in the 2s orbital. In the periodic table of the elements it is expected to.
Or the electronic configuration of Sodium is 1s 2 2s 2 2p 6 3s 1 since it contains a total of 11 electrons the first two-electron will go in the 1s orbital the next two in the 2s orbital the next six electrons in the 2p orbital and the remaining one electron will go in 3s orbital. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively which are used until the element is discovered confirmed and a permanent name is decided upon. A hybrid quantum-classical algorithm for solving many-electron problems is developed enabling the simulation with the aid of 16 qubits on a quantum processor of chemical systems with up to 120.
The p-orbital can have a maximum of six electrons. An atom of boron atomic number 5 contains five electrons. Carbon exhibits 4 2 -4 oxidation state.
The s shaped orbital is able to hold 2 electrons the p shaped orbital can hold 6 electrons the d orbital can hold 10 and finally the f orbital can hold 14 electrons. Earth is the third planet from the Sun and the only astronomical object known to harbor lifeWhile large volumes of water can be found throughout the Solar System only Earth sustains liquid surface waterAbout 71 of Earths surface is made up of the ocean dwarfing Earths polar ice lakes and riversThe remaining 29 of Earths surface is land consisting of continents and. The first two electrons of neon enter the 1s orbital and the next two electrons enter the 2s orbital.
Which has been discussed in detail above. C 4 acquired the electron configuration of neon and it achieves a stable electron configuration. The Lewis structure of carbon dioxide shows that when we say bonding electrons we need to count the lines on the structureOne line corresponds to two electrons.
Must contain at least 4 different symbols. Atoms can jump from one orbital to another orbital in the excited state. It has one of the highest electronegativities among the elements 304 on the Pauling scale exceeded only by chlorine.
We know that the element in group-18 is heliumHe. It formed 46 billion years ago from the gravitational collapse of a giant interstellar molecular cloudThe vast majority 9986 of the systems mass is in the Sun with most of the remaining mass contained in the planet JupiterThe four inner system planetsMercury Venus Earth and. We already know that the p-subshell has three orbitals.
So the remaining six electrons enter the 2p orbital. Where X is any atom or molecule X is. The first two electrons of aluminum enter the 1s orbital.
The s-orbital can have a maximum of two electrons. 6 to 30 characters long. In atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals.
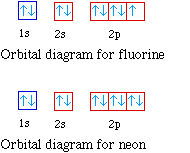
The ground-state electron configuration of fluorine is 1s 2 2s 2 2p 5. Therefore the neon full electron configuration will be 1s 2 2s 2 2p 6. Mercury is the smallest planet in the Solar System and the closest to the SunIts orbit around the Sun takes 8797 Earth days the shortest of all the Suns planets.
The three major constituents of Earths atmosphere are nitrogen oxygen and argonWater vapor accounts for roughly 025 of the atmosphere by mass. He neonNe argonAr kryptonKr xenonXe and radonRn. To create an orbital diagram of an atom you first need to know Hunds principle and Paulis exclusion principle.
IDM Members meetings for 2022 will be held from 12h45 to 14h30A zoom link or venue to be sent out before the time. To write the orbital diagram of heliumHe you have to do the electron configuration of helium. Therefore the nitrogen full electron configuration will be 1s 2 2s 2 2p 3.
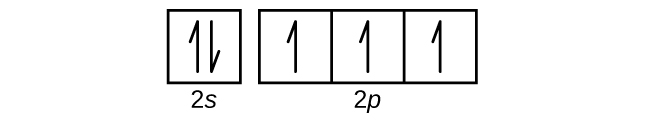
It features an extensive vocabulary and a. The orbitals are p x p y and p z and each orbital can have a maximum of two electrons. So the electron will enter the 4s orbital first and enter the 3d orbital when the 4s orbital is full.
The method of entering electrons into orbitals through the Aufbau principle is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. Therefore the next two electrons enter the 2s orbital. Term symbols with LS coupling.
Ununennium also known as eka-francium or element 119 is the hypothetical chemical element with symbol Uue and atomic number 119. For example the electron configuration of the neon atom is 1s 2 2s 2 2p 6 meaning that the 1s 2s and 2p subshells are occupied by 2 2 and 6 electrons respectively. The electron configuration shows that the orbit at the end of.
The Solar System is the gravitationally bound system of the Sun and the objects that orbit it. This glossary of chemistry terms is a list of terms and definitions relevant to chemistry including chemical laws diagrams and formulae laboratory tools glassware and equipmentChemistry is a physical science concerned with the composition structure and properties of matter as well as the changes it undergoes during chemical reactions. In physics and chemistry ionization energy IE American English spelling ionisation energy British English spelling is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom positive ion or molecule.
The oxidation state of the element changes depending on the bond formation. The nonbonding electrons on the.
Electronic Structure Of Atoms Electron Configurations General Chemistry I Course Hero

Create An Orbital Diagram For Nitrogen Neon Ppt Download

Electron Configuration For Iron Fe Fe2 And Fe3
How Many P Orbitals Are There In A Neon Atom Socratic

Create An Orbital Diagram For Nitrogen Neon Ppt Download

For Elements 1 36 There Are Two Exceptions To The Filling Order As Predicted From The Periodic Table Draw The Atomic Orbital Diagrams For The Two Exceptions And Indicate How Many Unpaired Electrons
Orbital Diagrams Total Element Electrons H 1 He 2 Li 3 Be 4 1s 2s Ppt Download

Neon Orbital Diagram Electron Configuration And Valence Electrons
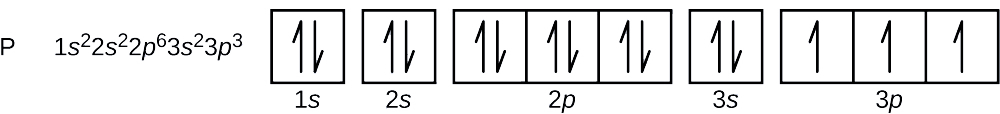
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts
Arrangements Of Electrons In The Orbitals Of An Atom Is Called Its Electron Configuration

How To Write The Orbital Diagram For Neon Ne Youtube

Neon Ne
Neon Ne Electron Configuration And Orbital Diagram
3 1 Electron Configurations Problems Chemistry Libretexts

How To Write The Orbital Diagram For Neon Ne Youtube
Neon Ne Electron Configuration And Orbital Diagram
Neon Orbital Diagram Electron Configuration And Valence Electrons